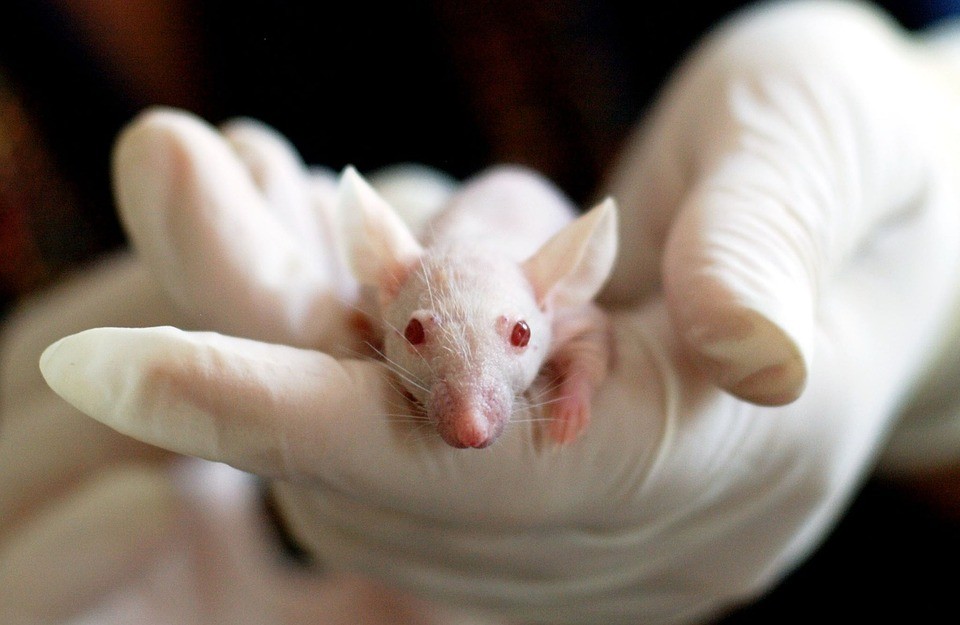
A very important part of the scientific process is testing it out, especially in the medical and chemical experiments.
Most organizations working in pharmaceutical products require that before releasing a new drug to have it tested on humans. To reach this point, it has to be deemed relatively safe by testing it on animal test subjects first.
It’s established that animal testing has been a stable of scientific research for a long time; however, is it really necessary? Are there any other alternatives? These questions must have crossed your mind as a scientific researcher.
In 1964, the Deceleration of Helsinki was released. Till this day, it’s considered to be one of the most influential acts governing research ethics on humans. The deceleration has been used as an aggregate of most of the rules and regulations that are related to laboratory tests on humans.
The deceleration also tackled the importance of publication ethics and its application to ensure the humane treatment of the test subjects.
That being said, is it the same when it comes to animal test subjects? How is it possible to conduct research on animal specimens in an ethical and humane way?
History of Animal Testing in Scientific Research
There is no definitive date or data detailing the first experiment on an animal for scientific purposes.
Animal research (or in vivo testing) is thought to have existed in parallel with the beginning of scientific experimentation. As a result, the debate of the humane ways of animal testing has existed since then, as well.
The British Royal Society states that most of our modern scientific discoveries saw the light of day thanks to animal testing in a way. Other parties in favor state that the practice of animal testing can’t be substituted and is necessary for the development of mankind.
The National Academy of Science in the United States argues that even with technological advancement, computer models can’t give authentic, organic results to carry in the stead of testing on biological life forms. Hence, the first phase of testing any medication is to ensure that it has passed laboratory tests on animals.
On the other hand, activists and organizations that are concerned with animal safety argue that there are plenty of other substitutions that can give the same results like animal testing. Some people even go on a limb in speculating that there are conspiracies of leaving these methods obscure in order to keep the trade of animals with laboratories booming.
It’s undeniable that testing on animals is necessary (pragmatically, regardless of the moral and ethical grounds). Yet, there are guidelines and ethics that surely work as a governing mechanism of the process.
Laws Governing Ethics of Experimenting on Animals
The international scientific society imposed some laws and regulations to ensure that the animals used in testing are treated humanely.
For example, in the United States, The Animal Welfare Act became active in 1966. It was agreed upon by President Lyndon Johnson. This is not the first law to tackle this issue, as it was preceded by the Cruelty to Animals Act 1876, issued by the British Parliament.
These acts are considered to be the founding stones to what we have now as legal mechanisms to protect animal welfare and rights in scientific testing, ensuring that test subjects are treated within the boundaries of scientific and legal ethics.
How to Employ These Ethics and Guidelines as a Researcher?
As a researcher, you want to consider the ethics at hand if your research requires experimentation on animals.
Afterward, you need to consider the following guidelines:
- Benefit: If the benefit to humanity and science is worth the price of testing on the specimen (which may lead to damage that can’t be rectified).
- Humane Testing Environment: Making sure that there is no suffering (as much as possible) induced on the specimens in the research.
- Euthanasia: After the tests are concluded, it has to be ensured that the animals with irreparable damaged are euthanized humanly and accordingly. The process must be conducted carefully and thoughtfully to avoid any unnecessary stress to the specimen.
- Ensuring The Use of Effective Species: You have to ensure that you are using the most prompt species suitable for your experiment. The type, whether invertebrates, vertebrates, etc…, must be considered to make sure there is less need to repeat the experiment (i.e., applying Reduction concept).
- Sedation: It’s a merit to make sure that the specimen is sedated accordingly during the testing to minimize distress (if it won’t impair the search results).
- Accumulation: If there are previous test results on a previous species, it’s a better practice to incorporate these data to decrease the amount of testing needed on the same species in your current experiment (e.g., if there are test results to an effective component X on guinea pigs, which acts as a part of your drug, you can use these results to rule out testing it again on your current guinea pig specimen).
Are There Other Alternatives to Animal Testing in Scientific Research?
Till now, scientists are working on alternatives to the process of testing on animals. A guiding protocol for testing on animals, dubbed The Three Rs, is applied in most countries when it comes to this matter.
The term was coined by W.M Russel and R.L Burch in 1959, stating that it’s appropriate to test on animals after considering:
- Replacement (avoid using animals in research altogether)
- Reduction (reducing the need for specimens to test effectively)
- Refinement (minimize pain and stress on the test subject)
As a researcher, if alternatives can achieve one of these criteria, it’s a merit to consider it.
It’s possible to use alternative methods to testing on animals; some mediums have been invented for this sole purpose and they cover the aforementioned criteria in some cases.
The effective range and results of these methods depend on the nature of the study. If it’s possible for one of them to be a candidate, it should be considered thoroughly.
- Organs-on-a-chip: Scientists in Harvard’s Wyss Institute created a product called organs-on-a-chip, acting as a small, controllable environment of cells that act like human cells; thus, it gives the room for testing the effects of experimental medications on the said organ. Yet, there are arguments that it doesn’t provide the necessary ecosystem of a biological life-form as a whole.
- Microdosing: Microdosing is a method of shifting the test on human volunteers by dosing them with miniature volumes of the drug or compound and researching its effects in a safe environment. Arguments against microdosing state that the effective dosage can’t be tested well and doesn’t provide the same accurate results as being tested on animals.
- Computer Simulation (In Silico): Simulation in a virtual environment can act as a viable substitute for animal testing if it will provide similar results with similar quality. However, it’s argued that virtual simulation can’t give feedback to all results that may take place in a variable, volatile biological ecosystem.
Yet if these alternatives provide the same results range and accuracy in a controlled environment, it should be incorporated instead.
Is It Necessary to Use Animals in Scientific Research?
It’s a fact that most of the scientific progress we achieved has been done thanks to the help of our little furry friends.
There will be always the need for testing in scientific and medical research; there will always be the need for a biological life form to be the subject of testing to ensure the medical research progresses.
As Machiavellian as it may sound, the benefit is far too great to shift to alternatives that won’t provide the same results.
Yet, that doesn’t mean that this evil shouldn’t be performed with some good in it…
Further Readings and Resources:
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2002542/
- http://www.bbc.co.uk/ethics/animals/using/experiments_1.shtml
- https://en.wikipedia.org/wiki/Animal_testing
- https://en.wikipedia.org/wiki/Animal_euthanasia
- https://olaw.nih.gov/
- https://web.archive.org/web/20090416104435/http://www.nal.usda.gov/awic/legislat/usdaleg1.htm
- http://www.understandinganimalresearch.org.uk/animals/a-z-animals/
- https://www.navs.org/what-we-do/keep-you-informed/legal-arena/research/explanation-of-the-animal-welfare-act-awa/#.XCdi4VUzbIU